Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:2 Percentile:76.52(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (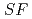
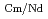 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
Hayashi, Hirokazu; Tsubata, Yasuhiro; Sato, Takumi
Nihon Genshiryoku Gakkai Wabun Rombunshi (Internet), 22(3), p.97 - 107, 2023/08
The Japan Atomic Energy Agency has chosen nitride fuel as the first candidate for the transmutation of long-lived minor actinides (MA) using accelerator-driven systems (ADS). The pyrochemical method has been considered for reprocessing spent MA nitride fuels, because their decay heat should be very large for aqueous reprocessing. This study was conducted to investigate the effect of decay heat on the pyrochemical reprocessing of MA nitride fuels. On the basis of the estimated decay heats and the temperature limits of the materials that are to be handled in pyrochemical reprocessing, quantities adequate for handling in argon gas atmosphere were evaluated. From these considerations, we proposed that an electrorefiner with a diameter of 26 cm comprising 12 cadmium (Cd) cathodes with a diameter of 4 cm is suitable. On the basis of the size of the electrorefiner, the number necessary to reprocess spent MA fuels from 1 ADS in 200 days was evaluated to be 25. Furthermore, the amount of Cd-actinides (An) alloy to produce An nitrides by the nitridation-distillation combined reaction process was proposed to be about one-quarter that of Cd-An cathode material. The evaluated sizes and required numbers of equipment support the feasibility of pyrochemical reprocessing for MA nitride fuels.
 (M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd
(M = Gd, Np) by the reaction of MN with Pd and chlorination of MPd using cadmium chloride
using cadmium chlorideHayashi, Hirokazu; Shibata, Hiroki; Sato, Takumi; Otobe, Haruyoshi
Journal of Radioanalytical and Nuclear Chemistry, 332(2), p.503 - 510, 2023/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)The formation of MPd (M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu
(M = Gd, Np) by the reaction of MN with Pd at 1323 K in Ar gas flow was observed. Cubic AuCu -type GdPd
-type GdPd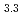 (
( = 0.4081
= 0.4081  0.0001 nm) and NpPd
0.0001 nm) and NpPd (
( = 0.4081
= 0.4081  0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi
0.0001 nm) were identified, respectively. The product obtained from the reaction of NpN with Pd contained additional phases including the hexagonal TiNi -type NpPd
-type NpPd . Chlorination of the MPd
. Chlorination of the MPd (M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl
(M = Gd, Np) samples was accomplished by the solid-state reaction using cadmium chloride at 673 K in a dynamic vacuum. Pd-rich solid solution phase saturated with Cd and an intermetallic compound PdCd were obtained as by-products of MCl formation.
formation.
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0502 (Internet), 2 Pages, 2022/04
This report summarizes the results obtained in FY2020 at the Electron Linac Facility of the University of Tokyo. The radiolysis process of  -hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
-hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kobayashi, Taishi*
The Chemistry of Thorium, Plutonium and MA, 254 Pages, 2022/03
The chemistry of nuclear materials such as Thorium (Part 1) and Plutonium (Part 2) was described in relation from the fundamentals on solid chemistry and solution chemistry to the practicals on the experiment and evaluation method in detail. Minor actinides such as Neptunium, Americium, Curium and Protoactinium, was introduced the basics on the solid and solution chemistry.
Murakami, Tsuyoshi*; Hayashi, Hirokazu
Journal of Nuclear Materials, 558, p.153330_1 - 153330_7, 2022/01
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Excess amounts of dissolution agents, CdCl and ZrCl
and ZrCl , are required to dissolve transuranium (TRU: Pu and minor actinides) nitrides into LiCl-KCl melts at the chemical dissolution step, which is the first step in the reprocessing of used nitride fuels. We propose an electrochemical process where the remaining Zr and Cd are recovered from the melts to be recycled as dissolution agents for the chemical dissolution step, leaving TRU in the melts. Since the initial concentration ratio of CdCl
, are required to dissolve transuranium (TRU: Pu and minor actinides) nitrides into LiCl-KCl melts at the chemical dissolution step, which is the first step in the reprocessing of used nitride fuels. We propose an electrochemical process where the remaining Zr and Cd are recovered from the melts to be recycled as dissolution agents for the chemical dissolution step, leaving TRU in the melts. Since the initial concentration ratio of CdCl /ZrCl
/ZrCl remaining in the melts would depend on the condition of the chemical dissolution step and would vary during the proposed electrochemical recovery process, electrochemical behaviors of Zr and Cd were investigated in LiCl-KCl melts with various concentration ratios of CdCl
remaining in the melts would depend on the condition of the chemical dissolution step and would vary during the proposed electrochemical recovery process, electrochemical behaviors of Zr and Cd were investigated in LiCl-KCl melts with various concentration ratios of CdCl /ZrCl
/ZrCl at 723 K to confirm the basic feasibility of the proposed process. Potentiostatic electrolysis was performed using a liquid Cd cathode at -1.05 V (vs. Ag/AgCl), which was a more positive potential than the redox potentials of TRU on the liquid Cd electrode. The obtained results showed that the current efficiency for recovering Zr and Cd from the melts was as high as 100% regardless of the CdCl
at 723 K to confirm the basic feasibility of the proposed process. Potentiostatic electrolysis was performed using a liquid Cd cathode at -1.05 V (vs. Ag/AgCl), which was a more positive potential than the redox potentials of TRU on the liquid Cd electrode. The obtained results showed that the current efficiency for recovering Zr and Cd from the melts was as high as 100% regardless of the CdCl /ZrCl
/ZrCl concentration ratio in the melts.
concentration ratio in the melts.
Sasa, Toshinobu
Kasokuki, 18(4), p.233 - 240, 2022/01
Lead bismuth eutectic alloy (LBE) is a promising option as a spallation target for accelerator-driven transmutation systems (ADS) to reduce the radiological toxicity from long-lived radioactive waste. LBE is a heavy metal and has suitable characteristics both as a spallation target and as a coolant for transmutation systems. However, LBE is also known as a highly corrosive with structural materials. In this paper, technological developments to overcome the issue, the latest research activities such as hightemperature operation and oxygen concentration control to ensure corrosion resistance, are introduced together with the outline of the target for ADS.
Kaneko, Masashi
Nihon Genshiryoku Gakkai-Shi ATOMO , 64(1), p.30 - 34, 2022/01
, 64(1), p.30 - 34, 2022/01
Partitioning of minor actinides from rare earths is one of the most important techniques to develop group separation of high-level radioactive liquid waste. In this issue, the results of prediction of separation performance between minor actinides and rare earths observed in solvent extraction and the separation mechanism by means of using density functional theory are explained.
Miyazaki, Yasunori; Sano, Yuichi
Hoshasen Kagaku (Internet), (112), p.27 - 32, 2021/11
no abstracts in English
Kaneko, Masashi; Sasaki, Yuji; Wada, Eriko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 50(10), p.1765 - 1769, 2021/10
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)Density functional theory calculation is applied to predict the stability constants for Eu and Am
and Am complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
 0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am
0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am selectivity over Eu
selectivity over Eu .
.
 -P for an advanced reprocessing
-P for an advanced reprocessingHoriuchi, Yusuke; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Kida, Fukuka*; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 330(1), p.237 - 244, 2021/10
Times Cited Count:6 Percentile:65.59(Chemistry, Analytical)Applicability of tetra2-ehylhexyl diglycolamide (TEHDGA) impregnated adsorbent for minor actinide (MA) recovery from high level liquid waste (HLLW) in extraction chromatography technology was investigated through batch-wise adsorption and column separation experiments. Distribution ratio of representative fission product elements were obtained by the batch-wise experiments, and TEHDGA adsorbent was shown to be preferable to TODGA adsorbent for decontamination of several species. All Ln(III) supplied into the TEHDGA adsorbent packed column was properly eluted from the column, and the applicability of the adsorbent was successfully showed by this study.
Sakamoto, Atsushi; Kibe, Satoshi*; Kawanobe, Kazunori*; Fujisaku, Kazuhiko*; Sano, Yuichi; Takeuchi, Masayuki; Suzuki, Hideya*; Tsubata, Yasuhiro; Ban, Yasutoshi; Matsumura, Tatsuro
JAEA-Research 2021-003, 30 Pages, 2021/06
Japan Atomic Energy Agency has been developing a solvent extraction process called SELECT to recover minor actinides (MA) from spent nuclear fuel. In the SELECT process, TDdDGA, HONTA, and ADAAM are used as the extractants for MA + Ln corecovery, MA/Ln separation and Am/Cm separation, respectively. These extractants do not contain phosphorus (P), and consist of carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). In this study, in order to give beneficial information for designing flowsheet, the mass transfer coefficients of Ln between HNO solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO
solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO / TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO
/ TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO / HONTA - n-dodecane system are under 10
/ HONTA - n-dodecane system are under 10 m/s.
m/s.
 /Eu
/Eu selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.
selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.Kaneko, Masashi; Sasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 58(5), p.515 - 526, 2021/05
Times Cited Count:3 Percentile:35.51(Nuclear Science & Technology)Density-functional theory calculations were applied to molecular structure and complex formation reaction modelings of metal ion complexes with diethylenetriaminepentaacetic acid (DTPA) and its bisamide (DTPABA) chelates to understand the metal ions selectivity between Am and Eu
and Eu . The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am
. The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am ion forms higher stable complexes with both chelates than Eu
ion forms higher stable complexes with both chelates than Eu ion, being consistent with the experimental results. The higher Am
ion, being consistent with the experimental results. The higher Am selectivity over Eu
selectivity over Eu was suggested to originate in the larger bond overlap between Am
was suggested to originate in the larger bond overlap between Am 5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am
5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am /Eu
/Eu selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
Iwasa, Toma; Takano, Masahide
JAEA-Technology 2020-024, 29 Pages, 2021/03
Partitioning and transmutation of minor actinides (MA) is an important issue to reduce volume and radio-toxicity of high-level radioactive wastes. In Nuclear Science Research Institute, we have been carrying out R&D on MA-bearing nitride fuel for accelerator driven system. In the actual nitride fuel fabrication process, a special nitrogen gas highly enriched with 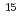 N is required to avoid
N is required to avoid  C production from
C production from  N by (n,p) reaction in the fuel. For the economical use of such expensive gas, we need a nitrogen circulation refining system that can remove carbon monoxide (CO) evolved by carbothermic nitridation of oxides and can use the nitrogen gas in the closed system without loss. To develop the system, at first we listed up the performance requirements, and then designed and assembled a prototype system for laboratory-scale demonstration. The system consists of CO removal unit and circulation unit that can automatically keep the system pressure and the gas flow rate constant. As a result of demonstration on the nitridation of oxide, both units completely satisfy the requirements. We confirmed that the concept can be applied to the actual fuel fabrication with further additional function such as automatic hydrogen feed for the control of decarburization.
N by (n,p) reaction in the fuel. For the economical use of such expensive gas, we need a nitrogen circulation refining system that can remove carbon monoxide (CO) evolved by carbothermic nitridation of oxides and can use the nitrogen gas in the closed system without loss. To develop the system, at first we listed up the performance requirements, and then designed and assembled a prototype system for laboratory-scale demonstration. The system consists of CO removal unit and circulation unit that can automatically keep the system pressure and the gas flow rate constant. As a result of demonstration on the nitridation of oxide, both units completely satisfy the requirements. We confirmed that the concept can be applied to the actual fuel fabrication with further additional function such as automatic hydrogen feed for the control of decarburization.
 -P column by neutron resonance absorption imaging
-P column by neutron resonance absorption imagingMiyazaki, Yasunori; Watanabe, So; Nakamura, Masahiro; Shibata, Atsuhiro; Nomura, Kazunori; Kai, Tetsuya; Parker, J. D.*
JPS Conference Proceedings (Internet), 33, p.011073_1 - 011073_7, 2021/03
Neutron resonance absorption imaging was adapted to observe the Eu band adsorbed in the CMPO/SiO -P column for minor actinide recovery by extraction chromatography. Several wet columns were prepared by either light water or heavy water and compared with the dry column to evaluate the neutron transmission. The neutron transmission spectra showed that 45% was transmitted through the dry column while 20% and 40% were transmitted through the wet columns of light water and heavy water, respectively. The results indicated that heavy water is more applicable than light water to observe the Eu adsorption band in the CMPO/SiO
-P column for minor actinide recovery by extraction chromatography. Several wet columns were prepared by either light water or heavy water and compared with the dry column to evaluate the neutron transmission. The neutron transmission spectra showed that 45% was transmitted through the dry column while 20% and 40% were transmitted through the wet columns of light water and heavy water, respectively. The results indicated that heavy water is more applicable than light water to observe the Eu adsorption band in the CMPO/SiO -P column.
-P column.
Sasa, Toshinobu; Saito, Shigeru; Obayashi, Hironari; Ariyoshi, Gen
JPS Conference Proceedings (Internet), 33, p.011051_1 - 011051_6, 2021/03
To realize Accelerator-driven system (ADS) for minor actinide transmutation, JAEA proposes to construct the Proton Irradiation Facility in J-PARC. The facility is planned to solve technical issues for safe application of Lead-bismuth Eutectic Alloy (LBE). The 250 kW LBE spallation target will be located in the facility to prepare material irradiation database by both proton and neutron irradiation in the temperature range for typical LBE-cooled ADS. Various studies for important technologies required to build the facilities are investigated such as oxygen concentration control, instruments development, remote handling techniques for target maintenance, and spallation target design. The large scale LBE loops for mock up the 250 kW LBE spallation target and material corrosion studies are also manufactured and applied to various experiments. The latest status of 250 kW LBE spallation target design works will be summarized.
Miyazaki, Yasunori; Sano, Yuichi; Okamura, Nobuo; Watanabe, Masayuki; Koka, Masashi*
QST-M-29; QST Takasaki Annual Report 2019, P. 72, 2021/03
no abstracts in English

Miyazaki, Yasunori; Adachi, Junichi*; Masuda, Ryotaro*; Gejo, Tatsuo*; Hoshino, Masamitsu*
Photon Factory Activity Report 2021 (Internet), 2 Pages, 2021/00
no abstracts in English
Nakamura, Satoshi; Kimura, Takahiro; Ban, Yasutoshi; Tsubata, Yasuhiro; Matsumura, Tatsuro
JAEA-Technology 2020-009, 22 Pages, 2020/08
Partitioning and transmutation technology division is planning to measure fission rate ratios that contribute to validate nuclear data of minor actinides (MA). For this purpose, MA sources for fission chambers were prepared using electrodeposition method. The radioactivity of each MA source was quantified, and its uncertainty was evaluated. Seven types of MA sources with different radioactivity were prepared using four nuclides of  Np,
Np,  Am,
Am,  Am, and
Am, and  Cm. A
Cm. A  Cm source solution of which radioactivity was quantified by isotope dilution method was used to prepare working standard sources of
Cm source solution of which radioactivity was quantified by isotope dilution method was used to prepare working standard sources of  Cm. The radioactivities were quantified as 1461 Bq, 2179 Bq, and 2938 Bq for
Cm. The radioactivities were quantified as 1461 Bq, 2179 Bq, and 2938 Bq for  Np sources, 1.428 MBq for
Np sources, 1.428 MBq for  Am source, 370.5 kBq and 89.57 kBq for
Am source, 370.5 kBq and 89.57 kBq for  Am sources, and 2.327 MBq for
Am sources, and 2.327 MBq for  Cm source with, the uncertainty of 0.35% (1
Cm source with, the uncertainty of 0.35% (1 ). This report summarizes the method for preparation and quantification of MA sources, and uncertainty evaluation.
). This report summarizes the method for preparation and quantification of MA sources, and uncertainty evaluation.